When trying to combat a problem as pervasive and deadly as the opioid epidemic, ensuring that the public is well-informed becomes crucial. But with drug manufacturers going to extreme lengths to market their products, including paying doctors to push opioids, misinformation makes that task considerably harder. One example of this is the marketing push behind abuse-deterrent opioids.
What Are Abuse-Deterrent Opioids?
The late 1990s saw Purdue Pharma introduce a “revolutionary” pain management drug known as OxyContin. Purdue alleged these semisynthetic opioid pills contained ingredients that released oxycodone gradually over approximately 12 hours, preventing the entire dose from releasing immediately and extending pain relief. Before long, people figured out ways to bypass the extended release chemicals, resulting in a much faster and intense high.
OxyContin abuse began drawing mainstream concern, prompting drug manufacturers to respond. Their solution was to introduce abuse-deterrent opioids, a new formulation of opioid pills that was touted as a way to prevent abuse.
Since their introduction, there have been a number of misconceptions surrounding abuse-deterrent opioids.
Misconception #1: Abuse-Deterrent Opioids Cannot be Misused
One would think that pills specifically marketed to prevent abuse would be effective at doing so, but this is not the case. While it’s true that the formulation makes it more difficult to crush or dissolve the pills, a five-minute Google search reveals numerous ways to bypass the safeguards.
Though ultimately, abuse-deterrent opioids only work on the assumption that the patient wants to inhale or inject the drug; in reality, the vast majority of abusers swallow opioid pills as received.
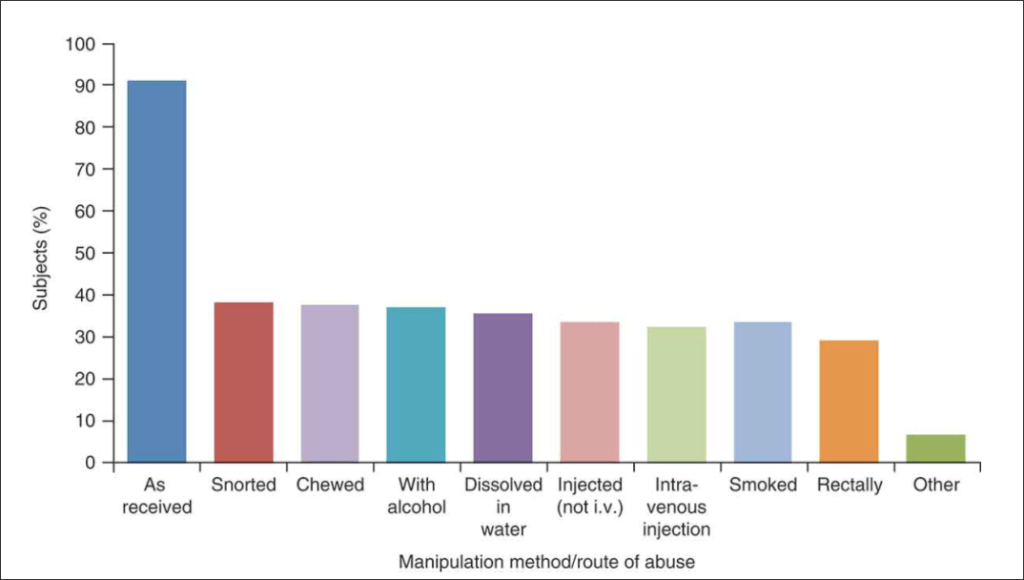
Courtesy of Taylor & Francis Online
Misconception #2: Abuse-Deterrent Opioids are Less Addictive
If you thought this misconception was true, you aren’t alone. A 2014 survey found that nearly one half of physicians believed that abuse-deterrent opioids were less addictive than their counterparts.
This simply isn’t true. The active ingredient in abuse-deterrent opioids is every bit as addictive as the non-abuse-deterrent version, and the fact that 46% of surveyed doctors believed otherwise is a massive problem in and of itself.
Misconception #3: Drug Manufacturers Developed Abuse-Deterrent Opioids to Help Curb the Opioid Epidemic
This one is a bit tricky, and requires some context. Back in 2007, Purdue Pharma agreed to pay $600 million in fines as part of a guilty plea for misleading regulators, doctors, and patients about OxyContin’s addictive properties and potential for abuse. Not only did this draw the public attention toward the dangers of opioids, but it also left the drug maker with an embarrassing black eye.
Purdue’s abuse-deterrent opioids received FDA approval in 2010, shortly before OxyContin’s patent was supposed to expire. Arguing that the original OxyContin formula was unsafe, Purdue successfully petitioned the FDA to remove the drug from the market, and reject any generic applications that lack abuse-deterrent qualities. The FDA complied with the petition, and announced it would no longer approve generic OxyContin applications only days before generic competition was set to reach the market.
This series of moves ensured that Purdue’s abuse-deterrent OxyContin would have no competition from generic OxyContin through 2027. While some may claim that the decision to bring an abuse-deterrent formula to the market was altruistic, there’s no denying the move greatly benefitted Purdue, while doing little to actually curb abuse.
Photo Credit: Jonathan Silverberg
0 Comments